REVIEW ARTICLE
Perspectives of Artificial Intelligence (AI) in Health Care Management: Prospect and Protest
Narmatha Sasi Prakash1, Lakshmi Chandran1, Madhana Kumar Sivakumar1, Ankul Singh Suresh Pratap Singh1, *
Article Information
Identifiers and Pagination:
Year: 2022Volume: 1
Issue: 2
E-location ID: e200922208961
Publisher ID: e200922208961
DOI: 10.2174/2666782701666220920091940
Article History:
Received Date: 02/05/2022Revision Received Date: 14/06/2022
Acceptance Date: 30/06/2022
Electronic publication date: 28/09/2022

Abstract
Background
Artificial intelligence postulates that computers will eventually supervise performing tasks through various pattern recognition with less or without human interventions and assistance. It appears to mimic human cognitive functions. Resembling the human brain, it receives various forms of raw data that are stored, aligned, surveyed, interpreted, analyzed, and converted to single processed data, making it easy to conclude and understand. Recently, in the digital world, machine learning, deep learning, neural network and AI applications are expanding widely, where humans have expertise.
Methods
A detailed literature survey was performed through an online database, such as ScienceDirect, Google Scholar, Scopus, Cochrane, and PubMed. The search keywords were Machine Learning OR Deep Learning OR Neural Networks OR Applications OR Pharmaceutical Innovations OR Technology OR Artificial Intelligence AND Pharmaceutical Sectors OR Clinical Pharmacology OR Healthcare OR Medical OR Pharmacovigilance OR Clinical Trials OR Regulatory OR Challenges. The literature search was limited to studies published in English.
Results
It was found that there is an immense growth of artificial intelligence in the sector of the pharmaceutical industry applied in drug discovery and drug development, clinical trials, and the pharmacovigilance sector. It has several clinical applications of AI as a tool in health care and biomedical research besides clinical practice. It also shows several challenges faced and methods to overcome them.
Conclusion
AI has great potential and future as a valuable tool in the healthcare and pharmaceutical industry by applying a scientific approach and averting real-life challenges.
1. INTRODUCTION
Artificial intelligence (AI) is a science that has been studied for over 50 years. John McCarthy invented the term in 1956 to describe the idea that computers could someday learn to execute tasks through pattern recognition with little to no human intervention. In computer science, AI studies “intelligent agents,” or systems that “perceive their environment and take actions to maximize their chances of achieving some goal.” Siri on Apple's iPhone, Netflix, Alexa on Amazon Echo, and other well-known examples are all made possible by AI [1, 2]. Artificial intelligence was first coined in the 1950s as a naive concept that computers could display human intellect. In the early 1960s, the first expert system, known as 'DENDRAL,' mechanized organic chemists' decision-making and problem-solving behavior with two main programs, Heuristic Dendral and MetaDendral. Artificial intelligence (AI) sought a place in healthcare and pharmaceuticals in the 1960s and 1970s [2].
Although progress is slow, some examples of where AI in health care include work done at UC Health in Colorado, where an AI-based scheduling tool is used to optimize surgical schedules, and many reports of incorrect medication administration or even surgery at the wrong site can be tracked down and followed up on, something that computers are particularly adapted [3]. People and scientists speculate that AI will be the herald of humanity's demise, ushering in a dystopian worldview in which robots rule the planet. On the other side, many people believe that wiser decision-making will lead to a massive boost in job prospects and economic growth [4]. In this article, we will describe both the potential and future of AI in the healthcare and pharmaceutical industry.
2. METHODS
2.1. Sources of Information and Search Strategies
A detailed literature survey was performed through an online database, such as ScienceDirect, Google Scholar, Scopus, Cochrane, and PubMed. The search keywords were Machine Learning OR Deep Learning OR Neural Networks OR Applications OR Pharmaceutical Innovations OR Technology OR Artificial Intelligence AND Pharmaceutical Sectors OR Clinical Pharmacology OR Healthcare OR Medical OR Pharmacovigilance OR Clinical Trials OR Regulatory OR Challenges. The literature search was limited to studies published in English.
2.2. Study Inclusion and Type of Intervention
The studies included various other studies involved with technology related to Artificial Intelligence and original articles conducted on Machine learning to assess the significance and basics of neuronal networks or technology-based interventions. Various studies on medical-based approaches and their health aspects were limited to the pharmaceutical sector.
3. MACHINE LEARNING
Artificial intelligence is a large area of study that includes a variety of technologies that function together. The following are some of the most critical technologies in healthcare: Machine learning, Neural networks, and deep learning [5]. ML is one of the most frequent types of AI. It is an analytical technique for fitting models in data or learning by training models using data, according to a 2018 Deloitte poll of 1,100 US managers whose companies were already exploring AI. It is a broad technique based on several AI approaches [5].
Traditional machine learning can be commonly utilized in precision medicine in the healthcare and pharmaceutical industries, forecasting which treatment protocols are likely to succeed on a patient based on numerous patient attributes and treatments. Most machine learning and precision medicine applications require supervised learning, which requires a training dataset with a predetermined outcome variable (e.g., illness onset) [6].
3.1. Neural Networks
A neural network is a more advanced sort of machine learning that has been employed in medical research for some time. Since the 1960s, it has been widely utilized. It was employed in a classification application to identify whether a patient is at risk of contracting a specific illness based on inputs, attributes, and other criteria [7]. It examines issues regarding inputs, outputs, and changeable weights or 'features' that connect them. Although the link to brain function is not powerful, it has been compared to the signaling mechanism of neurons [8].
Synapses that connect the dendrites of a biological neuron receive several impulses and transmit a single stream of action potentials through the axon. According to early theories, each neuron performs an essential cognitive function: it reduces complexity by classifying input patterns that impact artificial neural network models, which are made up of units that aggregate several inputs and generate a single output [9] (Fig. 1).
4. DEEP LEARNING
Deep learning is the most sophisticated kind of machine learning, which uses neural network models with multiple layers of variables to predict outcomes. The term “deep” refers to machine learning's multilayered structure. The CNNs (Convolutional neural networks) are the most promising DL technology in image recognition [10]. Deep learning in healthcare is frequently used to image potentially cancerous lesions in radiography. In the case of cancer, deep learning enables more precise imaging than prior generations of computer-aided detection (CAD) image analysis approaches [11]. Deep learning is gaining popularity in radionics (a branch of medicine that uses data characterization algorithms to extract a large number of quantitative information from medical images) or the discovery of clinically relevant data that the naked eye cannot discriminate. Thousands of hidden and unidentified data points may exist in such models that have yet to be identified [12] (Fig. 2).
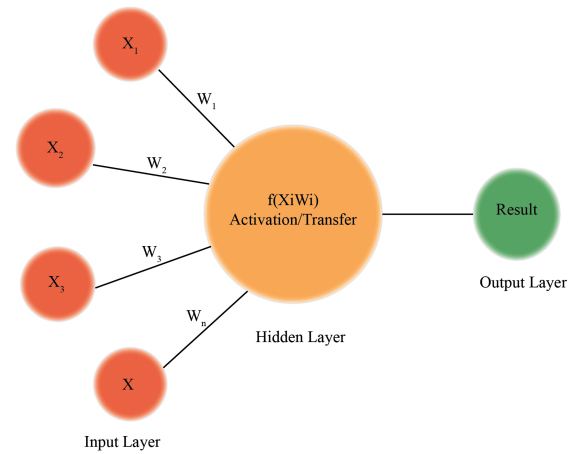 |
Fig. (1). Illustration of neural network. |
5. MACHINE LEARNING (ML) APPLICATIONS IN CLINICAL PHARMACOLOGY
Deep neural networks use a vast database of up to 192,284 drug-drug interactions to forecast drug-drug interactions and drug-food interactions for dietary recommendations, prescriptions, and novel compounds [13]. When it comes to individual safety, Daunhawer et al. employed machine learning to personalize safety in infants with hyperbilirubinemia [14]. Gaweda et al. also employed reinforcement learning to personalize pharmaceutical anemia therapy [15]. ML models recommend dose adjustments in real-time and could be extremely useful in customized healthcare [16].
ML has also demonstrated its value in bridging the gap between medication discovery and clinical development. For example, Hammann et al. used a decision tree method to predict the risk of Torsedes de Pointes from in vitro data, and Lancaster and Sobie used SVMs (Support Vector Machine Algorithm) to predict the risk of Torsedes de Pointes from in vitro data [17]. In a recent study, the ML-type control algorithm was combined with pharmacometricians' PK/PD models, resulting in a closed-loop control system [18].
6. CURRENT STATE OF AI IN THE PHARMA-CEUTICAL INDUSTRY
AI is gaining traction in various fields, including the pharmaceutical business. In this review, the use of AI in various pharmaceutical industry sectors is highlighted, including drug repurposing, drug discovery and development process, improving pharmaceutical productivity, and clinical trials; such implementation reduces human workload while also achieving targets in a short amount of time. AI in the pharmaceutical sector has enabled the industry to develop safe and effective treatments for previously untreatable ailments [19]. AI algorithms can drastically cut the cost and time it takes to discover and develop new drugs. AI has proven its value in pharmacovigilance by assisting in creating drug and biological toxicity databases [20]. Deep learning greatly influences science and technology in general, as well as drug development.
AI-driven technologies can be employed for drug repurposing in the case of neglected tropical diseases, which represent significant problems in terms of disease burden and death rate in underdeveloped and developing countries. These computational methods are rapid, making them excellent for screening huge libraries of available chemicals against specific molecular targets or disorders and repurposing current medications, clinical trials, and approved natural products [21]. Repurposing is advantageous since any leads discovered have already been evaluated for safety in people, allowing them to be tested in humans more rapidly and affordably than completely new medications [22].
AI has boosted the possibilities of leveraging huge data in pharmaceutical sciences, particularly machine learning technologies that allow computers to “learn” and perform jobs. The use of several computational approaches, such as molecular dynamics simulations and machine learning techniques, to forecast the water solubility of medicinal compounds has gained attention [23]. During the pre-formulation stage of the drug development process, the physicochemical characteristics of the drug material are assessed [24]. The stability, interaction with excipients, solubility, and bioavailability of a pharmaceutical substance are all influenced by its physicochemical properties. Transfer learning has shown to be a potential machine learning approach for further investigation in pre-formulation studies [25].
 |
Fig. (2). Components of deep learning algorithm. |
Artificial intelligence applications in drug discovery and design have significantly benefited from deep learning. In the United States, the Precision Medication Initiative was established to provide access to personalized medicine for the treatment of chronic disease conditions, such as cancer and diabetes [26]. SBDD (Structure-Based Drug Design) has proven instrumental in the rapid advancement of precision medicine. Computational modeling and simulation methods have been widely employed to develop new biomarkers, forecast potential intercellular pathways, and analyze protein conformational changes and cell activity [27]. Molecular dynamics simulation is a prominent computational tool for characterizing flexible binding sites and routes, kinetics, and thermodynamics, as well as understanding macromolecular conformational changes and their biological function, which might signal potential pathogenic processes [28] (Fig. 3).
7. PREDICTABLE FUTURE OF AI IN THE PHARMA INDUSTRY
7.1. Power of Artificial Intelligence (AI) in Healthcare, Research, and Clinical Practice
7.1.1. AI in Drug Discovery
AI can distinguish between hit and lead compounds, making it easier to validate therapeutic targets and optimize the structural design. Recently developed AI approaches, such as DL and relevant modeling studies, can be utilized to evaluate the safety and efficacy of pharmaceutical drugs based on massive data modeling and analysis [29, 30]. In 2012, Merck sponsored a QSAR ML competition to study the benefits of DL in drug discovery in the pharmaceutical industry [19]. Deep Learning models exhibited significant predictability compared to classic ML techniques for (ADMET) Absorption, Distribution, Metabolism, Excretion, and Toxicity [31]. QSAR modeling tools, such as linear discriminant analysis (LDA), have been used to identify possible drug candidates and have evolved into AI-based QSAR methodologies, support vector machines (SVMs), random forest (RF), and decision trees, which can be applied to speed up QSAR analysis [32-34] (Fig. 4).
Transformers are a newer generation of context-based models that extract representations from sequences using attention mechanisms and self-supervision. Transformers can predict drug-target interactions, model protein sequences, and retrosynthetic reactions [23]. Due to the advances made in the natural language processing domain, the COVID-19 pandemic has cleared the way for advancements in sequence-based models, such as genomics, proteomics, and transcriptomics [35, 36]. When trained on molecules or protein sequences, recurrent neural networks (RNNs) and long short-term memory (LSTM) networks have successfully shown the ability to predict secondary structure, quantitative structure-activity relationship (QSAR) modeling, and function prediction [37].
7.1.2. AI in Drug Screening
Developing a new medicine can take a decade and cost money. Therapeutic medicines fail Phase II clinical trials and regulatory approval in most cases. Nearest-Neighbor classifiers, RF, extreme learning machines, SVMs, and deep neural networks (DNNs) are some methods used in VS to predict in vivo activity and toxicity [38]. Using a genetic algorithm variable selection technique with SVMs, a study has created QSAR models for predicting vascular endothelial growth factor receptor (VEGFR) 2 inhibition by aminopyrimidine-5-carbaldehyde-oxime derivatives [39].
 |
Fig. (3). Applications of AI in the pharmaceutical industry. |
 |
Fig. (4). Current trend logarithm for deep learning techniques. |
DNNs can frequently produce better future predictions on a group of big heterogeneous QSAR data sets, according to studies based on Merck's Drug Discovery initiative [40]. It has been noticed that great generalization capacity and powerful feature extraction ability of deep learning methods have been used in predicting chemical attributes and synthesizing the required compounds, which will further encourage the use of AI in the drug creation field [41-43].
7.1.3. Challenges in the Regulatory Environment
With the integration of AI technologies into healthcare, a slew of ethical issues arises. One such ethical challenge in clinical treatment concerns amplifying biases in existing data, regardless of individual clinical interactions. Despite the knowledge that concomitant asthma exacerbates pneumonia, AI categorized patients with pneumonia alone as high-risk but wrongly classified those with pneumonia plus asthma as low-risk [44, 45]. Health records and data are usually unreliable. Despite data purification and standardization, unforeseen loopholes will emerge, posing a threat to the quality of datasets used to train AI systems [46]. Patient data privacy is another significant issue [47]. Data is collected in real-time, and it is examined in real-time for patterns in the care process, procedures that can be improved, and other underlying patterns like different patient reactions to differential treatments [48]. Finally, these discoveries are frequently integrated into the clinical treatment process. In this setting, data leakage and data privacy concerns occur [49]. Data-driven innovations may be stifled as a result of health privacy violations [50]. As a result, politicians and regulators must collaborate with physicians, patients, and manufacturers to develop a new framework for regulation that harnesses the power of emerging technologies while ensuring appropriate security and privacy of personal data [51].
Enhanced data security standards must accompany regulations to avoid stifling progress in the industry. These range from improved data encryption per client to the adoption of federated learning, which allows models to be trained centrally despite data being spread across several clients [46, 52, 53]. The FDA has called for adopting “agile” regulatory methods for software used in medicine to accommodate the quicker rate of development and potential for innovation in software-based devices. While machine learning promises efficiency, price savings, and improved health results, regulation of this rapidly growing sector must explicitly address and call out health disparities rather than omitting how these technologies may have significant positive and negative consequences for underrepresented groups [54].
7.1.4. AI in Clinical Trials
The last five years have seen an exponential increase in digital technology penetration into clinical trials. In clinical trials, real-world data, such as electronic health records (EHRs) and clinical trial findings, varies from incorporating AI in diagnostic devices to using real-world data, such as EHRs and clinical trial outcomes [55]. EHRs are useful data sources for comparative effectiveness research and novel trial designs that can aid in resolving important clinical challenges while increasing efficiency and cutting costs. The first experience with EHRs has been positive, and as more information becomes available, the application of EHRs for clinical research will continue to evolve [56-59].
The USFDA has recently cleared the way for many biopharmaceutical companies in the USA to conduct AI-empowered virtual clinical trials. It also licensed crowd-sourced clinical trial designs and metrics that can be digitally communicated to reduce the overall expenditure [60]. Although using AI and other new digital technologies can compromise participant privacy and confidentiality, regulatory agencies and IRBs have issued guidance on using digital tools [55].
7.1.5. AI in Pharmacovigilance and Drug Safety
The purpose of pharmacovigilance is to ensure the safety of patients exposed to therapeutic medications during clinical trials and research. The most significant activities in the PV sector are the detection and reporting of ADRs, the technical coding of adverse effects, the development of safety individual reports, the assessment of severity, and the linkage with suspected pharmaceuticals [61]. All of these rely on time-consuming human interference; therefore, ADR detection needs the creation of new technology. Traditionally, pharmacovigilance is based on a clinical examination of case reports gathered by recognized organizations [62]. Data mining algorithms are created to enhance this process by allowing assessors to sift through enormous amounts of data and concentrate on topics essential to public health [63, 64].
AI and ML can make the pharmacovigilance process simpler and more effective in a variety of ways, including a) Reduced cycle times, which allows for more automatic processing, b) Improving the quality and accuracy of the data, c) Managing various types of incoming data formats, and d) Identifying ADRs [62]. Combining text mining methodologies [65] with rule-based and specific machine-learning classifiers, the US Vaccine Adverse Event Reporting System [66] highlights the feasibility of building effective medical text classifiers for spontaneous reporting systems [67].
8. AI IN CLINICAL PRACTICE AND HEALTH CARE
Possible benefits of AI are described in Table 1 [68-75].
8.1. Challenges
- Although AI can potentially transform medical practice, there are several technological challenges to solve. As machine learning relies significantly on high-quality training data, collecting data representative of the target patient population is critical [52, 76].
- When there is an unsatisfactory inter-expert agreement on a diagnostic task, consensus diagnoses have been demonstrated to significantly increase the performance of machine-learning models trained on the data. When dealing with heterogeneous data, proper data curation is required. Furthermore, attaining the gold standard of a patient's clinical status necessitates clinicians individually reviewing their clinical notes, which is prohibitively expensive on a population scale [77, 78].
- Sophisticated algorithms that can address the quirks and noises of different datasets will improve the predictability of the models and, therefore, the safety of using them in life-or-death choices [79, 80].
- Several high-performing machine-learning models produce impossible outcomes for humans to understand without assistance [81].
- Artificial intelligence (AI) could potentially replace some healthcare employees and clinicians in basic tasks, reshaping the healthcare workforce and changing the present reimbursement paradigm [82, 83].
8.2. Overcoming the Challenges
To overcome challenges, AI researchers and physicians must collaborate to prioritize and develop applications that address critical clinical requirements. Hospital administrators must assess and limit clinical workflow disturbance when adopting new AI applications [52, 84]. Companies will need to figure out the best framework for conducting prospective clinical trials to assess the efficacy of AI systems in clinical settings. Insurers should evaluate the value of medical AI systems and, if necessary, alter their payment policies to decrease healthcare costs while enhancing quality [85]. To facilitate the development and implementation of medical AI applications, multidisciplinary and multi-sector collaborations will be required [86].
9. SCIENTIFIC APPROACH
The healthcare system, like water, should adjust to changes in space and technology. It should not be stopped; instead, it should educate itself via its own experiences and strive to make ongoing advances in practice [87]. Today's age has progressed to the point where it is the most significant issue to alter and correct all potential flaws. Healthcare “systems thinking” considers the traditional interactions between patients and physicians and larger-scale organizations and cycles [88] (Fig. 5).
| Types of Diseases | Clinical Application of AI | Refs. |
|---|---|---|
| Retinal disease | We can identify, localize, and quantify pathogenic characteristics in practically every macular and retinal disease using machine learning (ML) and, in particular, deep learning (DL). | [68] |
| Ovarian cancer | Preoperative investigations can predict the pathology diagnosis of ovarian cancer. | [69] |
| Cardiovascular disease | Prediction of cardiovascular event risk. | [70] |
| Chronic obstructive lung diseases | The application of machine learning in the automated interpretation of pulmonary function tests for the differential diagnosis of obstructive lung disorders has been proven successful. In computed tomography, deep learning algorithms, such as convolutional neural networks, are state-of-the-art for finding obstructive patterns. | [71] |
| Gastrointestinal diseases | Application study in pathology, imaging, and endoscopic diagnoses for gastrointestinal illnesses using convolutional neural networks (CNNs). | [72] |
| Liver diseases | Clinical applications include detecting and analyzing localized liver lesions, simplifying treatment, and predicting hepatic treatment response in medical imaging of liver illnesses. | [73] |
| Renal diseases | Artificial intelligence is already being utilized to improve clinical care, hemodialysis prescriptions, and the prediction of increasing immunoglobulin deficiency. | [74] |
| Respiratory diseases | AI and machine learning are used to diagnose interstitial lung disease, and a few more papers were found on mechanical ventilation, chest X-ray interpretation, and bronchial asthma diagnosis. | [75] |
 |
Fig. (5). Potential applications of AI in healthcare research. |
Before using AI systems in healthcare, they must be “trained” using data generated from clinical activities, such as clinical laboratory, treatment, assignment medical notes, screening, electronic recordings from medical devices, physical examinations, diagnosis and images, etc., so that they can learn about similar groupings of subjects, subject-feature connections, and desired results [89, 90]. AI is successful when it contains a machine learning component for managing structured data and a natural language processing component for unstructured mining text. The implementation of AI in real life continues to face challenges. The first stumbling point is the limitations [91]. Under existing legislation, there are no standards to assess AI systems' safety and efficacy. Data exchange is a second stumbling block [92]. Apart from ethical problems, the main roadblock is that this novel feature of medical care necessitates a standardized, comparative assessment of the impact of robotic systems on health indicators, as well as assessments of changes in psychological and physical condition, side effects, and outcomes.
CONCLUSION
The pharmaceutical sector faces various complex difficulties, including increasing medication and therapy prices, and society demands considerable changes in this area. With the application of AI in pharmaceutical product manufacture, personalized medications with the requisite dosage, release characteristics, and other relevant factors may be considered according to specific patient demands. Using the maximum up-to-date AI based technology will now no longer simply shorten the time it takes for merchandise to attain the market, but it is going to additionally enhance product quality and safety of common production procedures, in addition to higher useful resource utilization and cost-effectiveness, emphasizing the significance of automation. We believe that AI has a bright future in healthcare and will favor medical practice.
AI can be useful in every step of drug development. ML and DL algorithms can bring about a shift in the paradigm of various intricacies of healthcare. AI in healthcare can potentially improve preventative care and overall quality of life. It can result in more precise treatment plans and, as a result, improved patient results. In clinical trials, AI-powered technologies may help determine the safety and efficacy of a medicinal product, as well as the best market positioning and pricing. Strict laws for the standardization of such data-driven advances are required to ensure the privacy and security of patient data. With the introduction of AI into the sector, there is a growing risk that numerous present employments may be lost. Hence, it should be implemented to reduce such losses and strain on healthcare providers. Shortly, AI is anticipated to become a valuable tool in the healthcare and pharmaceutical industries.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study's conception and design. Material preparation and data collection were performed by Madhana Kumar S, Narmatha S, Lakshmi Chandran, and Ankul Singh S. The first draft of the manuscript was written by Narmatha S, and all authors commented on previous versions. The analysis of the article was performed by Ankul Singh S. All authors read and approved the final manuscript.
LIST OF ABBREVIATIONS
| ADMET | = Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| AI | = Artificial Intelligence |
| CNN's | = Convolutional Neuronal Networks |
| DL | = Deep Learning |
| DNNs | = Deep Neuronal Networks |
| EHRs | = Electronic Health Records |
| FDA/ USFDA | = United States Food and Drug Administration |
| IRBs | = Institutional Review Board |
| LDA | = Linear Discriminant Analysis |
| LSTM | = Long Short-term Memory |
| ML | = Machine Learning |
| PK/PD | = Pharmacokinetic/ Pharmacodynamic |
| PV | = Pharmacovigilance |
| QSAR ML | = Quantitative Structure-activity Relationship Machine Learning |
| RF | = Random Forest |
| RNNs | = Recurrent Neural Networks |
| SBDD | = Structure-based Drug Design |
| SVMs | = Support Vector Machine Algorithm |
| VEGFR | = Vascular Endothelial Growth Factor Receptor |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to all those with whom they have had the pleasure to work during this and other related projects.
REFERENCES
| [1] | Henstock PV. Artificial intelligence for pharma: Time for internal investment. Trends Pharmacol Sci 2019; 40(8): 543-6. |
| [2] | Helm JM, Swiergosz AM, Haeberle HS, et al. Machine learning and artificial intelligence: Definitions, applications, and future directions. Curr Rev Musculoskelet Med 2020; 13(1): 69-76. |
| [3] | Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: What do these terms mean and how will they impact health care? J Arthroplasty 2018; 33(8): 2358-61. |
| [4] | Haeberle HS, Helm JM, Navarro SM, et al. Artificial intelligence and machine learning in lower extremity arthroplasty: A review. J Arthroplasty 2019; 34(10): 2201-3. |
| [5] | Davenport T, Kalakota R. The potential for artificial intelligence in healthcare. Future Healthc J 2019; 6(2): 94-8. |
| [6] | Badillo S, Banfai B, Birzele F, et al. An intro-duction to machine learning. Clin Pharmacol Ther 2020; 107(4): 871-85. |
| [7] | Shahid N, Rappon T, Berta W. Applications of artificial neural networks in health care organizational decision-making: A scoping review. PLoS One 2019; 2: e0212356. |
| [8] | Brown N, Cambruzzi J, Cox PJ, Davies M, Dunbar J, Plumbley D. Big data in drug discovery. In: Progress in Medicinal Chemistry 2018; 57: 277-356. |
| [9] | Kriegeskorte N, Golan T. Neural network models and deep learning. Curr Biol 2019; 29(7): R231-6. |
| [10] | Sarker IH. Deep learning: A comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci 2021; 2(6): 420. |
| [11] | Choy G, Khalilzadeh O, Michalski M, et al. Current applications and future impact of machine learning in radiology. Radiology 2018; 288(2): 318-28. |
| [12] | Scapicchio C, Gabelloni M, Barucci A, Cioni D, Saba L, Neri E. A deep look into radiomics. Radiol Med (Torino) 2021; 126(10): 1296-311. |
| [13] | Ryu JY, Kim HU, Lee SY. Deep learning improves prediction of drug–drug and drug–food interactions. Proc Natl Acad Sci USA 2018; 115(18): E4304-11. |
| [14] | Daunhawer I, Kasser S, Koch G, et al. Enhanced early prediction of clinically relevant neonatal hyperbilirubinemia with machine learning. Pediatr Res 2019; 86(1): 122-7. |
| [15] | Gaweda AE, Muezzinoglu MK, Aronoff GR, Jacobs AA, Zurada JM, Brier ME. Individualization of pharmacological anemia management using reinforcement learning. Neural Netw 2005; 18(5-6): 826-34. |
| [16] | Schork NJ. Artificial intelligence and personalized medicine.Precision Medicine in Cancer Therapy Cancer treatment and research2019; 178. [https://doi.org/10.1007/978-3-030-16391-4_11] |
| [17] | Hammann F, Gutmann H, Vogt N, Helma C, Drewe J. Prediction of adverse drug reactions using decision tree modeling. Clin Pharmacol Ther 2010; 88(1): 52-9. |
| [18] | McComb M, Bies R, Ramanathan M. Machine learning in pharmacometrics: Opportunities and challenges. Br J Clin Pharmacol 2022; 88(4): 1482-99. |
| [19] | Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today 2021; 26(1): 80-93. |
| [20] | Cáceres EL, Tudor M, Cheng AC. Deep learning approaches in predicting ADMET properties. Future Med Chem 2020; 12(22): 1995-9. |
| [21] | Winkler DA. Use of artificial intelligence and machine learning for discovery of drugs for neglected tropical diseases. Front Chem 2021; 9: 614073. |
| [22] | Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol 2011; 162(6): 1239-49. |
| [23] | Gupta R, Srivastava D, Sahu M, Tiwari S, Ambasta RK, Kumar P. Artificial intelligence to deep learning: machine intelligence approach for drug discovery. Mol Divers 2021; 25(3): 1315-60. |
| [24] | Jones TM. Preformulation studies. Pharmaceutical Formulation 2018; 1-41. Available from: http://ebook.rsc.org/ |
| [25] | Damiati SA. Digital Pharmaceutical Sciences. AAPS PharmSciTech 2020; 21(6): 206. |
| [26] | Bohr A, Memarzadeh K. The rise of artificial intelligence in healthcare applications. Artificial Intelligence in Healthcare 2020; 25-60. |
| [27] | Lounnas V, Ritschel T, Kelder J, McGuire R, Bywater RP, Foloppe N. Current progress in Structure-Based Rational Drug Design marks a new mindset in drug discovery. Comput Struct Biotechnol J 2013; 5(6): e201302011. |
| [28] | Wang C, Xu P, Zhang L, Huang J, Zhu K, Luo C. Current strategies and applications for precision drug design. Front Pharmacol 2018; 9: 787. |
| [29] | Selvaraj C, Chandra I, Singh SK. Artificial intelligence and machine learning approaches for drug design: challenges and opportunities for the pharmaceutical industries. Mol Divers 2022; 26: 1893-913. [https://link.springer.com/10.1007/s11030-021-10326-z] |
| [30] | Vijayan RSK, Kihlberg J, Cross JB, Poongavanam V. Enhancing preclinical drug discovery with artificial intelligence. Drug Discov Today 2022; 27(4): 967-84. |
| [31] | Guan L, Yang H, Cai Y, et al. ADMET-score – a comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2019; 10(1): 148-57. |
| [32] | Dara S, Dhamercherla S, Jadav SS, Babu CHM, Ahsan MJ. Machine learning in drug discovery: A review. Artif Intell Rev 2022; 55(3): 1947-99. |
| [33] | Soufan O, Ba-alawi W, Magana-Mora A, Essack M, Bajic VB. DPubChem: a web tool for QSAR modeling and high-throughput virtual screening. Sci Rep 2018; 8(1): 9110. |
| [34] | Golbraikh A, Wang XS, Zhu H, Tropsha A. Predictive QSAR modeling: Methods and applications in drug discovery and chemical risk assessment. Handbook of Computational Chemistry 2016; 1-48. |
| [35] | Keshavarzi Arshadi A, Webb J, Salem M, Cruz E, Calad-Thomson S, Ghadirian N. Artificial intelligence for COVID-19 drug dis-covery and vaccine development 2020. |
| [36] | Yang X, Wang Y, Byrne R, Schneider G, Yang S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem Rev 2019; 119(18): 10520-94. |
| [37] | Bullock J, Luccioni A, Hoffman Pham K, Sin Nga Lam C, Luengo-Oroz M. Mapping the landscape of Artificial Intelligence applica-tions against COVID-19. J Artif Intell Res 2020; 69: 807-45. |
| [38] | Carracedo-Reboredo P, Liñares-Blanco J, Rodríguez-Fernández N, et al. Fer-nandez-Lozano, C. A review on machine learning approaches and trends in drug discovery. Comput Struct Biotechnol J 2021; 19: 4538-58. |
| [39] | Nekoei M, Mohammadhosseini M, Pourbasheer E. QSAR study of VEGFR-2 inhibitors by using genetic algorithm-multiple linear regressions (GA-MLR) and genetic algorithm-support vector machine (GA-SVM): a comparative approach. Med Chem Res 2015; 24(7): 3037-46. |
| [40] | Ma J, Sheridan RP, Liaw A, Dahl GE, Svetnik V. Deep neural nets as a method for quantitative structure-activity relationships. J Chem Inf Model 2015; 55(2): 263. |
| [41] | Vamathevan J, Clark D, Czodrowski P, et al. Ap-plications of machine learning in drug discovery and development. Nat Rev Drug Discov 2019; 18(6): 463-77. |
| [42] | Mayr A, Klambauer G, Unterthiner T, et al. Large-scale compari-son of machine learning methods for drug target prediction on ChEMBL. Chem Sci (Camb) 2018; 9(24): 5441-51. |
| [43] | Lavecchia A. Machine-learning approaches in drug discovery: methods and applications. Drug Discov Today 2015; 20(3): 318-31. |
| [44] | Rashid MBMA. Artificial Intelligence Effecting a Paradigm Shift in Drug Development. SLAS Technol 2021; 26(1): 3-15. |
| [45] | Jiang L, Wu Z, Xu X, et al. Opportunities and challenges of artificial intelligence in the medical field: current application, emerging problems, and problem-solving strategies. J Int Med Res 2021; 49(3) |
| [46] | Aung YYM, Wong DCS, Ting DSW. The promise of artificial intelligence: A review of the opportunities and challenges of artifi-cial intelligence in healthcare. Br Med Bull 2021; 139(1): 4-15. |
| [47] | Price WN II, Cohen IG. Privacy in the age of medical big data. Nat Med 2019; 25(1): 37-43. |
| [48] | Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst 2014; 2(1): 3. |
| [49] | Abouelmehdi K, Beni-Hessane A, Khaloufi H. Big healthcare data: preserving security and privacy. J Big Data 2018; 5(1): 1. |
| [50] | Cheng L, Liu F, Yao DD. Enterprise data breach: causes, challenges, prevention, and future directions. Wiley Interdiscip Rev Data Min Knowl Discov 2017; 7(5): e1211. |
| [51] | Hassan S, Dhali M, Zaman F, Tanveer M. Big data and predictive analytics in healthcare in Bangladesh: regulatory challenges. Heliyon 2021; 7(6): e07179. |
| [52] | Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelli-gence. BMC Med 2019; 17(1): 195. |
| [53] | Murphy K, Di Ruggiero E, Upshur R, et al. Artificial intelligence for good health: a scoping review of the ethics literature. BMC Med Ethics 2021; 22(1): 14. |
| [54] | Ferryman K. Addressing health disparities in the Food and Drug Administration’s artificial intelligence and machine learning regulatory framework. J Am Med Inform Assoc 2020; 27(12): 2016-9. |
| [55] | Rosa C, Marsch LA, Winstanley EL, Brunner M, Campbell ANC. Using digital technologies in clinical trials: Current and future applications. Contemp Clin Trials 2021; 100: 106219. |
| [56] | Cowie MR, Blomster JI, Curtis LH, et al. Electronic health records to facilitate clin-ical research. Clin Res Cardiol 2017; 106(1): 1-9. |
| [57] | Lee S, Xu Y, D’Souza AG, et al. Unlocking the potential of electronic health records for health research. Int J Popul Data Sci 2020; 5(1): 1123. |
| [58] | Lin WC, Chen JS, Chiang MF, Hribar MR. Applications of artificial intelligence to electronic health record data in ophthalmology. Transl Vis Sci Technol 2020; 9(2): 13. |
| [59] | Bennett WL, Bramante CT, Rothenberger SD, et al. Patient recruitment into a multicenter clinical cohort linking electron-ic health records from 5 health systems: Cross-sectional analysis. J Med Internet Res 2021; 23(5): e24003. |
| [60] | Inan OT, Tenaerts P, Prindiville SA, et al. Digitizing clinical trials. NPJ Digit Med 2020; 3(1): 101. |
| [61] | Suke SG, Kosta P, Negi H. Role of Pharmacovigilance in India: An overview. Online J Public Health Inform 2015; 7(2): e223. |
| [62] | Medhi B, Murali K, Kaur S, Prakash A. Artificial intelligence in pharmacovigilance: Practical utility. Indian J Pharmacol 2019; 51(6): 373-6. |
| [63] | Harpaz R, DuMouchel W, Shah NH, Madigan D, Ryan P, Friedman C. Novel data-mining methodologies for adverse drug event discovery and analysis. Clin Pharmacol Ther 2012; 91(6): 1010-21. |
| [64] | Kolling ML, Furstenau LB, Sott MK, et al. Data mining in healthcare: Applying strategic intelligence techniques to depict 25 years of research development. Int J Environ Res Public Health 2021; 18(6): 3099. |
| [65] | Chopard D, Treder MS, Corcoran P, et al. Text mining of adverse events in clinical trials: Deep learning approach. JMIR Med Inform 2021; 9(12): e28632. |
| [66] | Botsis T, Nguyen MD, Woo EJ, Markatou M, Ball R. Text mining for the vaccine adverse event reporting system: Medical text classification using informative feature selection. J Am Med Inform Assoc 2011; 18(5): 631-8. |
| [67] | Schmider J, Kumar K, LaForest C, Swankoski B, Naim K, Caubel PM. Innovation in pharmacovigilance: Use of artificial intelli-gence in adverse event case processing. Clin Pharmacol Ther 2019; 105(4): 954-61. |
| [68] | Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res 2018; 67: 1-29. |
| [69] | Akazawa M, Hashimoto K. Artificial intelligence in ovarian cancer diagnosis. Anticancer Res 2020; 40(8): 4795-800. |
| [70] | Krittanawong C, Virk HUH, Bangalore S, et al. Machine learning prediction in cardiovascular diseases: a meta-analysis. Sci Rep 2020; 10(1): 16057. |
| [71] | Das N, Topalovic M, Janssens W. Artificial intelligence in diagnosis of obstructive lung disease. Curr Opin Pulm Med 2018; 24(2): 117-23. |
| [72] | Zhao Y, Hu B, Wang Y, Yin X, Jiang Y, Zhu X. Identification of gastric cancer with convolutional neural networks: A systematic review. Multimedia Tools Appl 2022; 81(8): 11717-36. |
| [73] | Zhou LQ, Wang JY, Yu SY, et al. Artificial intelligence in medical imaging of the liver. World J Gastroenterol 2019; 25(6): 672-82. |
| [74] | Niel O, Bastard P. Artificial intelligence in nephrology: Core concepts, clinical applications, and perspectives. Am J Kidney Dis 2019; 74(6): 803-10. |
| [75] | Mekov E, Miravitlles M, Petkov R. Artificial intelligence and machine learning in respiratory medicine. Expert Rev Respir Med 2020; 14(6): 559-64. |
| [76] | Ahmed Z, Mohamed K, Zeeshan S, Dong X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database (Oxford) 2020; 2020: baaa010. |
| [77] | Thomasian NM, Kamel IR, Bai HX. Machine intelligence in non-invasive endocrine cancer diagnostics. Nat Rev Endocrinol 2022; 18(2): 81-95. |
| [78] | Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artifi-cial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ 2020; 368: l6927. |
| [79] | Yang G, Ye Q, Xia J. Unbox the black-box for the medical explainable AI via multi-modal and multi-centre data fusion: A mini-review, two showcases and beyond. Inf Fusion 2022; 77: 29-52. |
| [80] | Sarker IH. Machine Learning: Algorithms, real-world applications and research directions. SN Comput Sci 2021; 2(3): 160. |
| [81] | Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng 2018; 2(10): 719-31. |
| [82] | Ahuja AS. The impact of artificial intelligence in medicine on the future role of the physician. PeerJ 2019; 7: e7702. |
| [83] | Amann J, Vetter D, Blomberg SN, et al. To explain or not to explain?—Artificial intel-ligence explainability in clinical decision support systems. PLOS Digit Heal 2022; 1(2): e0000016. |
| [84] | Giordano C, Brennan M, Mohamed B, Rashidi P, Modave F, Tighe P. Accessing artificial intelligence for clinical decision-making. Front Digit Heal 2021; 3. |
| [85] | Gerke S, Babic B, Evgeniou T, Cohen IG. The need for a system view to regulate artificial intelligence/machine learning-based soft-ware as medical device. NPJ Digit Med 2020; 3(1): 53. |
| [86] | Secinaro S, Calandra D, Secinaro A, Muthurangu V, Biancone P. The role of artificial intelligence in healthcare: a structured litera-ture review. BMC Med Inform Decis Mak 2021; 21(1): 125. |
| [87] | Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S. High-quality health systems in the sustainable devel-opment goals era: Time for a revolution. Lancet Glob Health 2018; 6(11): e1196-252. |
| [88] | Meskó B, Drobni Z, Bényei É, Gergely B, Győrffy Z. Digital health is a cultural transformation of traditional healthcare. mHealth 2017; 3: 38-8. |
| [89] | Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support sys-tems: benefits, risks, and strategies for success. NPJ Digit Med 2020; 3(1): 17. |
| [90] | Lysaght T, Lim HY, Xafis V, Ngiam KY. AI-assisted decision-making in healthcare. Asian Bioeth Rev 2019; 11(3): 299-314. |
| [91] | Harrer S, Shah P, Antony B, Hu J. Artificial intelligence for clinical trial design. Trends Pharmacol Sci 2019; 40(8): 577-91. |
| [92] | Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol 2017; 2(4): 230-43. |




